Directed evolution—a laboratory technique that mimics natural selection—allows scientists to evolve genes and the proteins they encode. Traditionally, this technique has been used in microbes, mammalian cells, or in test tubes.
Now, however, researchers led by Prof. GAO Caixia from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences (CAS) and Prof. QIU Jinlong from the Institute of Microbiology of CAS have developed a pioneering system that enables rapid and scalable directed evolution of diverse genes directly in plant cells.
The platform, named Geminivirus Replicon-Assisted in Planta Directed Evolution (GRAPE), was published as a first release in Science on October 2 (10.1126/science.ady2167).
Modern agricultural production requires abundant genetic resources. Directed evolution can rapidly generate genetic variants with new and enhanced properties. However, efficient platforms for performing such evolution directly in plant cells have been lacking. One major challenge is the slow cell division rate in plants, which limits the speed of selection cycles and the enrichment of functional variants.
To address this challenge, we harnessed geminiviruses—plant DNA viruses that replicate DNA rapidly in plant cells via rolling circle replication (RCR). By linking the function of gene variants to the RCR of an artificial geminivirus replicon, we achieved selective amplification of desirable gene variants directly in plant cells.
Building on this approach, we developed GRAPE. In this platform, genes of interest (GOIs) are first mutagenized in vitro, and the resulting variants are inserted into artificial geminivirus replicons. The replicon libraries are then delivered into Nicotiana benthamiana leaves, where the desired gene activity is linked to viral replication. Variants that promote replication are enriched, while those that inhibit replication are depleted. Remarkably, a full selection cycle can be completed on a single leaf within four days.
Using GRAPE, we evolved the nucleotide-binding domain leucine-rich repeat-containing (NLR) immune receptor NRC3 to evade inhibition by the nematode effector SPRYSEC15 while preserving its immune activity. Iterative evolution of the rice NLR immune receptor Pikm-1 yielded variants that respond to six alleles of the Magnaporthe oryzae effector AVR-Pik, significantly expanding its recognition range. This strategy yields valuable genetic resources for breeding disease-resistant crops.
Compared with previous microbe-based systems, GRAPE offers distinct advantages: It excels at evolving GOIs responsible for plant-specific phenotypes (like disease resistance) or requiring plant-specific regulation, and it works directly within plant cells, eliminating the need for re-optimization. GRAPE can potentially evolve any gene functionally coupled to RCR. Beyond plant biology, GRAPE also holds promise for broader applications, such as evolving proteases to cleave specific targets for plant and pharmaceutical research.
GRAPE offers a rapid, efficient, and versatile platform that can accelerate plant synthetic biology and molecular breeding, opening new avenues for crop engineering and improving agricultural sustainability.
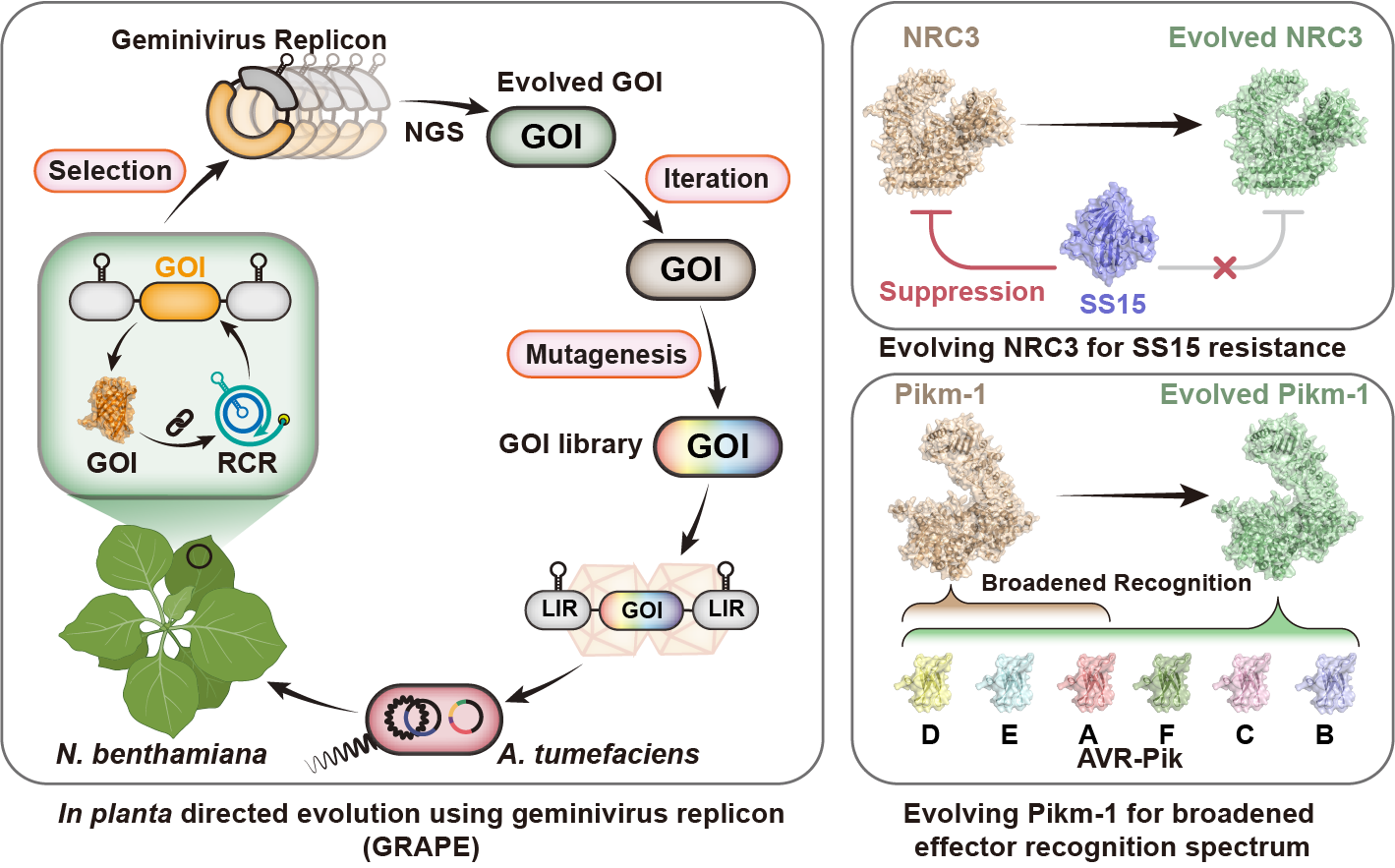
Figure. Graphical representation of GRAPE and its applications
附件:
